Follow the link and select ‘Models for infection studies’ and ‘Mouse’
Feel free to specify INFRAFRONTIER in your application.
This ISIDORe Open Call provides extensive user support to the selected projects for assessing novel potential vaccine and treatment compounds for COVID-19 in preclinical mouse models.

With more than 6 million deaths worldwide, the COVID-19 pandemic is one of the deadliest diseases to plague mankind in recent times. Considerable scientific effort from all over the world has been rallied to fight this common foe.
With this free-of-charge TA Call “COVID-19 BSL3 Pipeline”, INFRAFRONTIER is providing researchers access to test their innovative and novel COVID-19 therapeutics in a standardised infection pipeline that uses preclinical models to study COVID-19 infection.
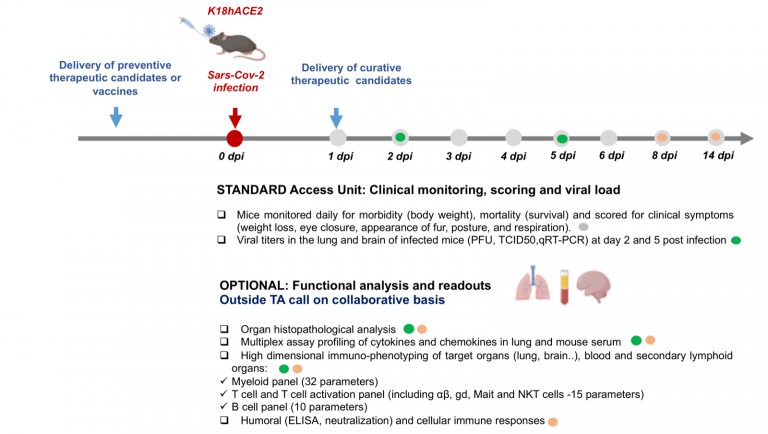
Main objective of this ISIDORe Trans-national Access Call is to provide extensive user support to the selected projects for assessing novel potential vaccine and treatment compounds for COVID-19. As starting material for the TA service, only sufficient amounts of the therapeutic candidate and desired mode of administration will be provided by the TA call users. It will be incumbent upon users to provide these materials and information. Viral particles, COVID-19 mouse models, cohort production and BSL3 pipeline analysis will be provided by INFRAFRONTIER partner – CIPHE. Further functional analysis and advanced readouts like organ histopathological analysis, multiplex assay profiling of cytokines and chemokines in lung and mouse serum etc. can be provided on a collaborative basis outside the scope of this TA call. A final infection profile report will be provided as a deliverable.
Application costs: This TA Call is free-of-charge for the selected applicants.
Please contact
Project Funding: This free-of-charge open call is funded by the ISIDORe project as part of the EU Research and Innovation programme Horizon Europe (Grant Number: 101046133).

Feel free to specify INFRAFRONTIER in your application.
Additional Information: Access Modalities
What is a Trans-national Access (TA) activity?
TA activities provide trans-national access to researchers or research teams to one or more infrastructures among those operated by INFRAFRONTIER partners. These access activities are implemented in a coordinated way such as to improve the overall services available to the research community. Access is made available to external users, either in person (‘hands-on’) or through the provision of remote scientific services, such as the provision of reference materials or samples or analysis of samples.
Who can participate in a Trans-national Access call?
Proposals for this Trans-national Access call can be submitted from non-commercial applicants around the world. Only 20% of the access units will be allocated to users that are not EU-member states or EU-associated states.
Is the TA service completely free-of-charge?
This TA service is covered by the ISIDORe project (Horizon Europe). The conditions for free access are 1) the user must work in an institution of a EU-Member State or EU-Associated State, 20% of access units will be allocated to international users, and 2) the user must be selected by the INFRAFRONTIER Evaluation Committee. The access to the COVID-19 therapeutic pipeline service is free-of-charge. However, the shipment of the therapeutic compound to CIPHE must be borne by the applicants.
Are submitted proposals treated with confidentiality?
Submitted project proposals will be collected and passed on by the ISIDORe Project Office to members of the external Evaluation Committee. All members of the Evaluation Committee signed a confidentiality agreement.
What is the selection process and the criteria used to assess submitted proposals?
Proposals from eligible customers for free-of-charge access to the ISIDORe COVID-19 BSL3 Pipeline Service will be subject to a review procedure. Contact